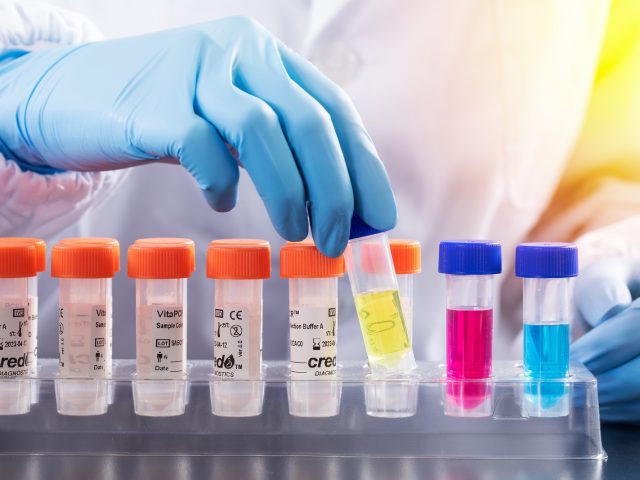
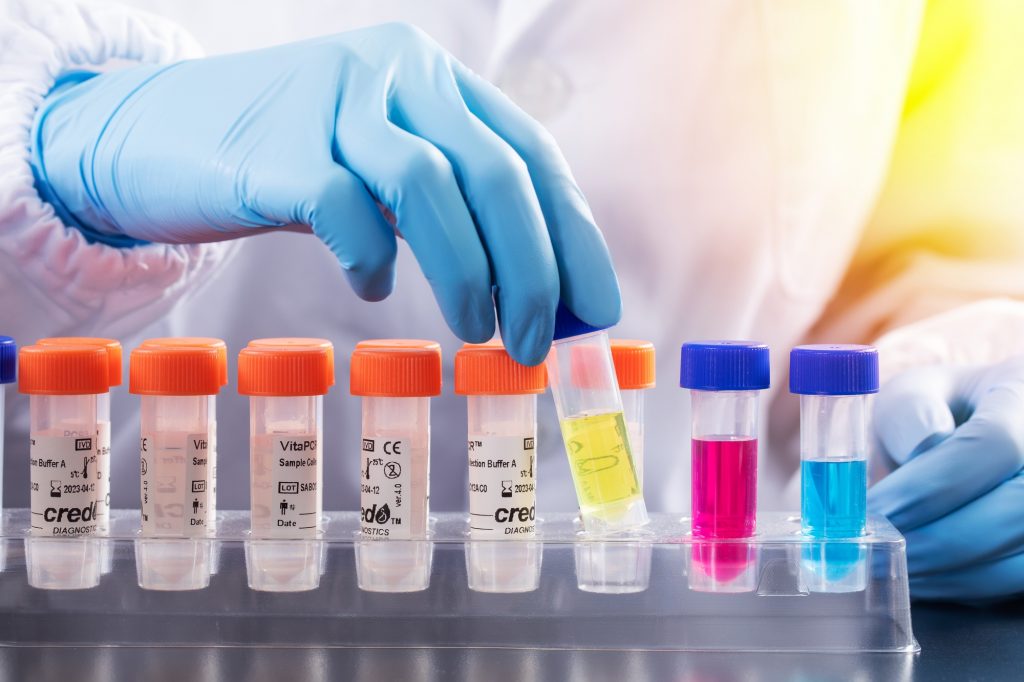
AT A FRACTION OF THE COST
into empowering people
Technology For All

Making better and faster informed decisions
Accelerate diagnostics turnaround time at a fraction of the cost
Turning informed decisions into empowering people
Diagnostics with rapid and uncompromising accuracy


Turning informed decisions into empowering people
Diagnostics with rapid and uncompromising accuracy

Providing advanced medical technology for all
Redefining healthcare for human clinical management

Laboratory Professional
Healthcare
Provider

Health System Administrator
ABOUT CREDO DIAGNOSTICS
Knowledge Empowering People
Since 2011, Credo Diagnostics has been dedicated to researching and developing revolutionary diagnostic technologies. We have created, manufactured, and commercialized accurate, rapid, and affordable molecular diagnostic solutions for point-of-care testing.

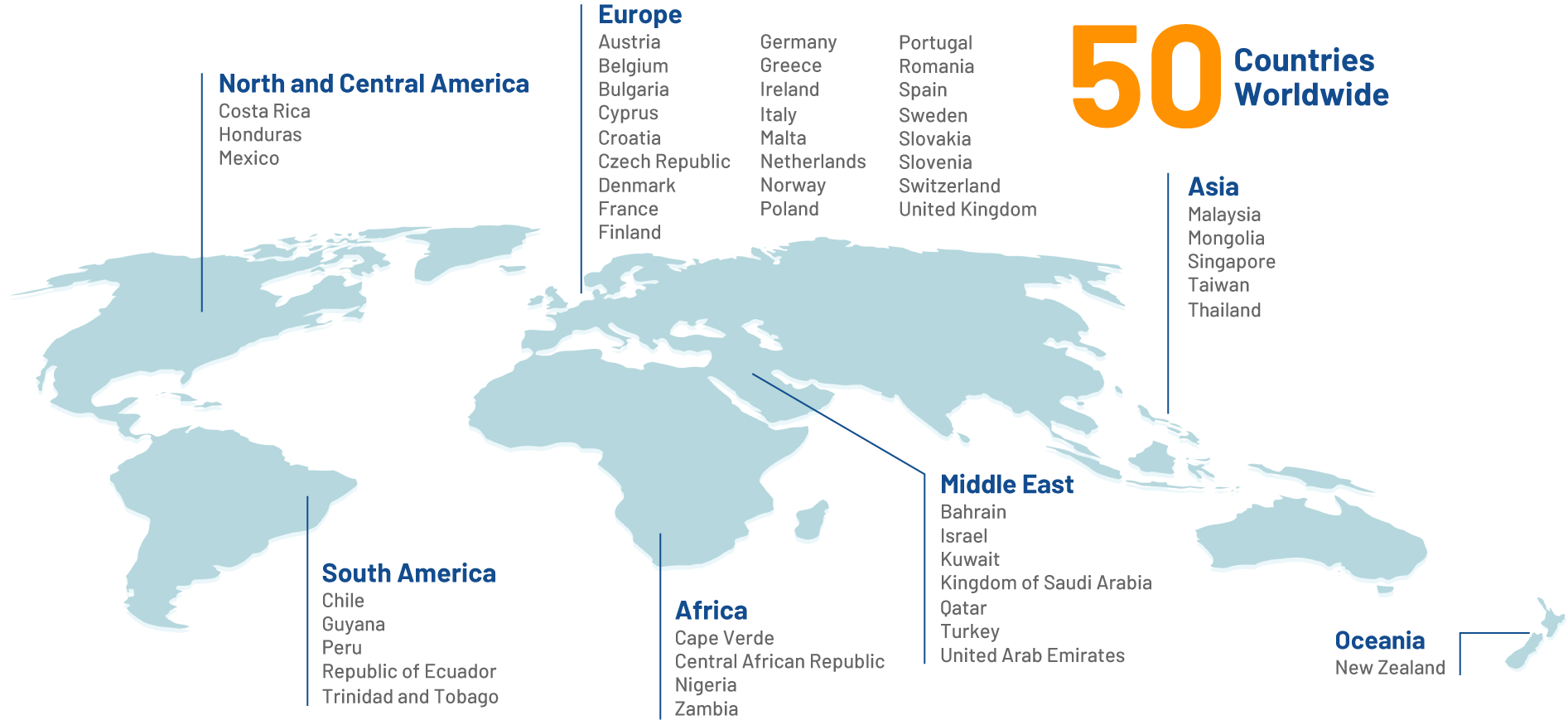
OUR REACH
Making a positive impact on the lives of billions of people around the globe
LATEST
Media News & Product Updates Keep Abreast with the Latest
Asian Flush and Easily Drunk: The Genetics of Alcohol Intolerance
Can you handle your liquo

Credo Preps MDx System, Multiplex Respiratory Test for US Launch
NEW YORK – Singapore-base

Miniaturized Real-Time PCR systems for SARS-CoV-2 detection at the Point-of-Care
VitaPCR™ RT-PCR test reac
Asian Flush and Easily Drunk: The Genetics of Alcohol Intolerance
Can you handle your liquo
Update on the Detection of the latest SARS-CoV-2 Variant-LP.8.1
The Level of Action: Gree