A Rapid and Accurate Diagnostic Test for Influenza & COVID-19 Detection at the Point-of-Care
In the post-pandemic era, it has become critical to distinguish between influenza and COVID-19 during flu season. However, due to the similar symptoms caused, it is actually challenging for clinicians to identify solely based on the signs and symptoms. Besides, the conventional time-consuming molecular tests might also provide the window period for virus spread. Hence, rapid and accurate testing which simultaneously detects influenza virus and SARS-CoV-2 has become essential to provide a timely diagnosis and help doctors make precise medical decisions on the spot.
VitaPCR™ Influenza/SARS-CoV-2 (Flu/SC2) Assay
REGISTRATION STATUS
CE-marked (IVDD)


VitaPCR™ – Accurate diagnostics, whenever and wherever you need it.
Combining a simplified 1 minute hands-on time procedure to ensure seamless integration into practitioners’ workflow, a compact and reliable design without the need for extra equipment like refrigeration, and a proprietary RT-PCR technology allowing fast and accurate diagnostics; VitaPCR™ allows the detection of Influenza and SARS-CoV-2 in 20 minutes from sample to results, at the Point-of-Care.
VitaPCR™ empowers practitioners with the right information at the right time to make the right decision and provide the right course of treatment to the patients.
Usability
- Minimal Training Required
- No additional equipment needed
- Room Temperature Storage
Performance
- High Accuracy
- Short Turnaround Time
- Intuitive result interpretation with Ct value & fluorescence curve
Diagnostic Procedure in 4 Steps
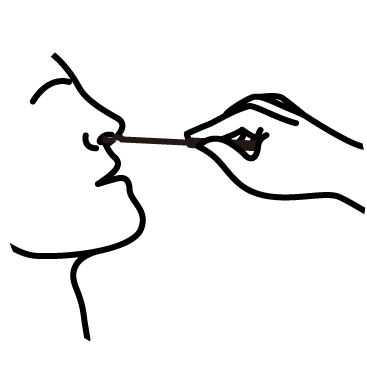
Collect
Collect sample

Lyse
Lyse the sample
thoroughly for 15 times

Transfer
Transfer the sample

Start Run
Results reported in
20 minutes

Collect
Collect sample

Lyse
Lyse the sample
thoroughly for 15 times

Transfer
Transfer the sample

Start Run
Results reported in
20 minutes
Intuitive Result Interpretation

VitaPCR™ Influenza/SARS-CoV-2 (Flu/SC2) Assay
Catalog No.
PCRAE0128
Technology
rRT-PCR
Quantity
20 tests/box
Targets
• M Gene – Influenza A
• NS Gene – Influenza B
• N Gene – SARS-CoV-2
Sample Types
Nasopharyngeal Swabs
Hands-on Time
< 1 min
Turnaround Time
20 mins
Kit Storage
5 – 25 °C / 41 – 77 °F

