SARS-CoV-2/Flu/RSV Assay
Symptoms of respiratory viral infections caused by SARS-CoV-2, Flu A/B, and RSV can be similar, making it challenging for clinicians to diagnose based on signs and symptoms alone.
The VitaSIRO solo™ SARS-CoV-2/Flu/RSV Assay is an advanced molecular diagnostic test designed for the automated VitaSIRO solo™ Instrument. This multiplex RT-PCR assay rapidly detects and differentiates SARS-CoV-2, influenza A and B, and RSV from nasal and nasopharyngeal swabs, supporting timely diagnosis and effective patient management in cases of overlapping respiratory symptoms.
VitaSIRO solo™ SARS-CoV-2 / Flu / RSV Assay

VitaSIRO solo™ – Molecular Diagnostics at Your Fingertips.
The VitaSIRO solo™ SARS-CoV-2/Flu/RSV Assay provides an efficient on-site solution for respiratory infection diagnosis, eliminating the need to ship samples to central labs and significantly reducing both costs and turnaround time. With a fully automated sample-to-result process, the VitaSIRO solo™ Instrument processes nasal and nasopharyngeal swabs, delivering reliable results for SARS-CoV-2, influenza A/B, and RSV within approximately 40 minutes.
Usability
- Multiplex Detection
- Ease of Use
- Room Temperature Storage
Performance
- Rapid Results
- High Sensitivity and Specificity
- Internal Control
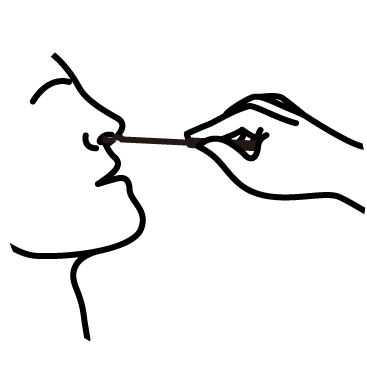
Diagnostic Procedure in 3 Steps

Collect
Collect sample

Transfer
Transfer the sample
to the cartridge

Start Run
Results reported in
40 minutes
Collect
Collect sample

Lyse
Lyse the sample
thoroughly for 15 times

Transfer
Transfer the sample

Start Run
Results reported in
20 minutes
Intuitive Result Interpretation

VitaSIRO solo™ SARS-CoV-2/Flu/RSV Assay
Catalog No.
PCRAD1102/PCRAD1112
Technology
Real-Time PCR + Fluorescence Spectrum Detection
Quantity
12 cartridge/box
Targets
SARS-CoV-2, Flu A/B, RSV A, RSV B
Specimen
Nasal Swab + Nasopharyngeal Swab
Hands-on Time
< 1 minute
Turnaround Time
40 mins
Kit Storage
4~30°C


