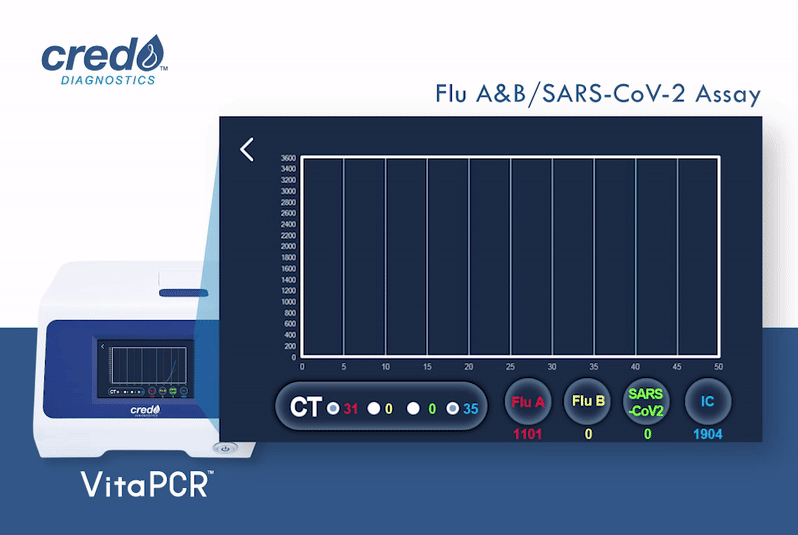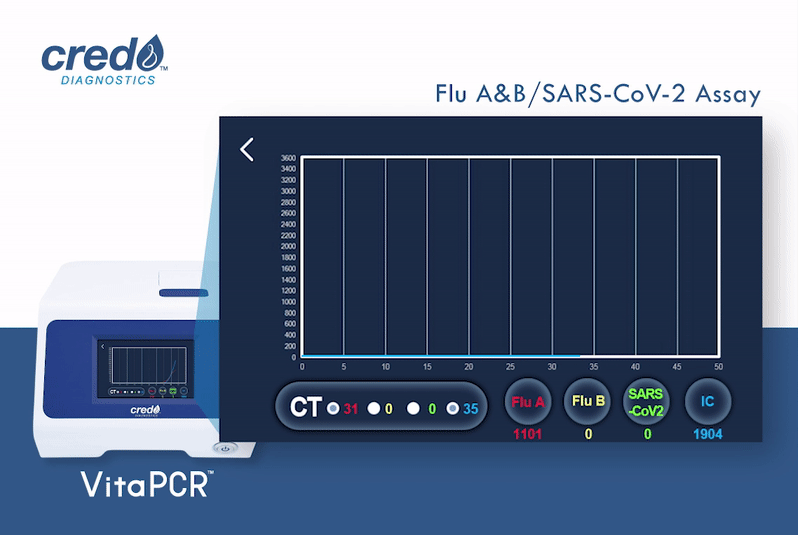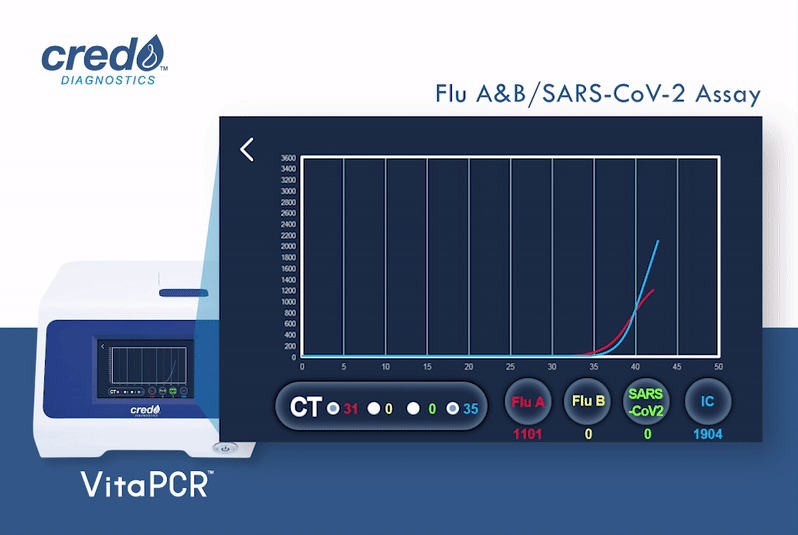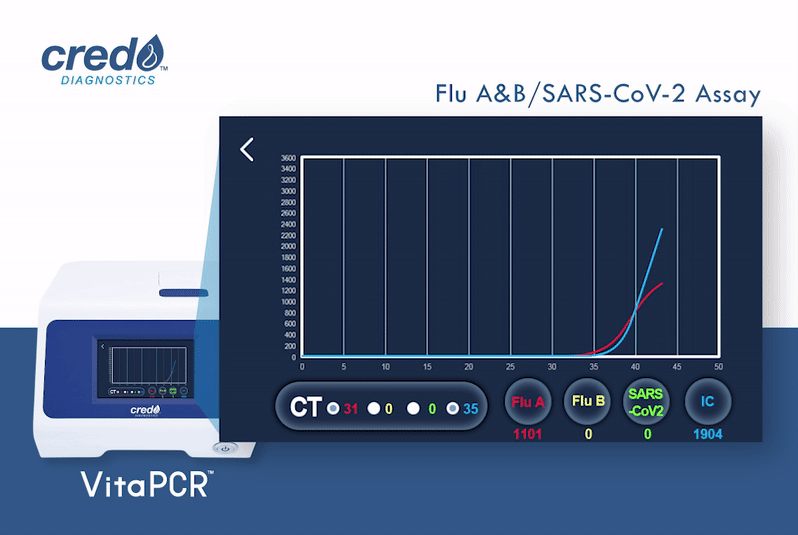
In response to the emergence of variants of influenza A H3N2 viruses, and to mitigate any potential risks that these variants may have on our assay performance, we, Credo Diagnostics Biomedical, have performed the BLASTn analysis (with the oligonucleotide primers and probes of the assays involving Influenza A virus detection) against the sequences of eight circulating influenza A variants (seven variants belong to clade 3C.2a1b.2a.2 and one variant belongs to clade 3C.2a1b.1a) listed in the Danish published study.¹
According to our analysis, we hereby confirm that all our Influenza A detection assays, including VitaPCR™ Flu A&B assay, VitaPCR™ Flu/RSV assay, and VitaPCR™ Influenza/SARS-CoV-2 (Flu/SC2) assay, are capable of detecting these eight variants correctly.