About Us

Today, doctors face the delicate challenge of making accurate diagnoses daily. However, the tools currently available to them are far from perfect. Medical practitioners often find themselves having to compromise between performance and convenience.
Since 2011, Credo Diagnostics has been researching and developing revolutionary diagnostic technologies. Our mission is to create, manufacture, and commercialize accurate, rapid, and affordable molecular diagnostic platforms that can be used at Point-of-Care.
We understand the importance of simplifying processes and speeding up diagnostic turnaround times, all while keeping costs low and ensuring the highest level of accuracy. That’s why we provide innovative solutions for diagnosis in the field of Human Health.
As a diagnostic company, we believe we have a moral responsibility and duty to doctors and patients. We strive to provide effective and rapid Point-of-Care Molecular Diagnostics that will redefine healthcare and revolutionize human clinical management.
Credo Diagnostics is committed to improving the tools available to doctors, enabling them to make more accurate diagnoses efficiently and affordably. We are dedicated to advancing healthcare through our innovative molecular diagnostic platforms, ultimately enhancing patient care and outcomes.

The Race For Flu Test Kits | VenturePreneurs | Full Episode
In 2020, Credo Biomedical debuted its rapid flu test in the international arena. This series looks at the challenges that fledgling ventures face if they choose to go global and why some companies take the leap. Follow the founders of each company as they navigate key goals and examine whether they succeed in meeting the real-time challenges of product development and sales that they set for themselves. Click thumbnail to watch.
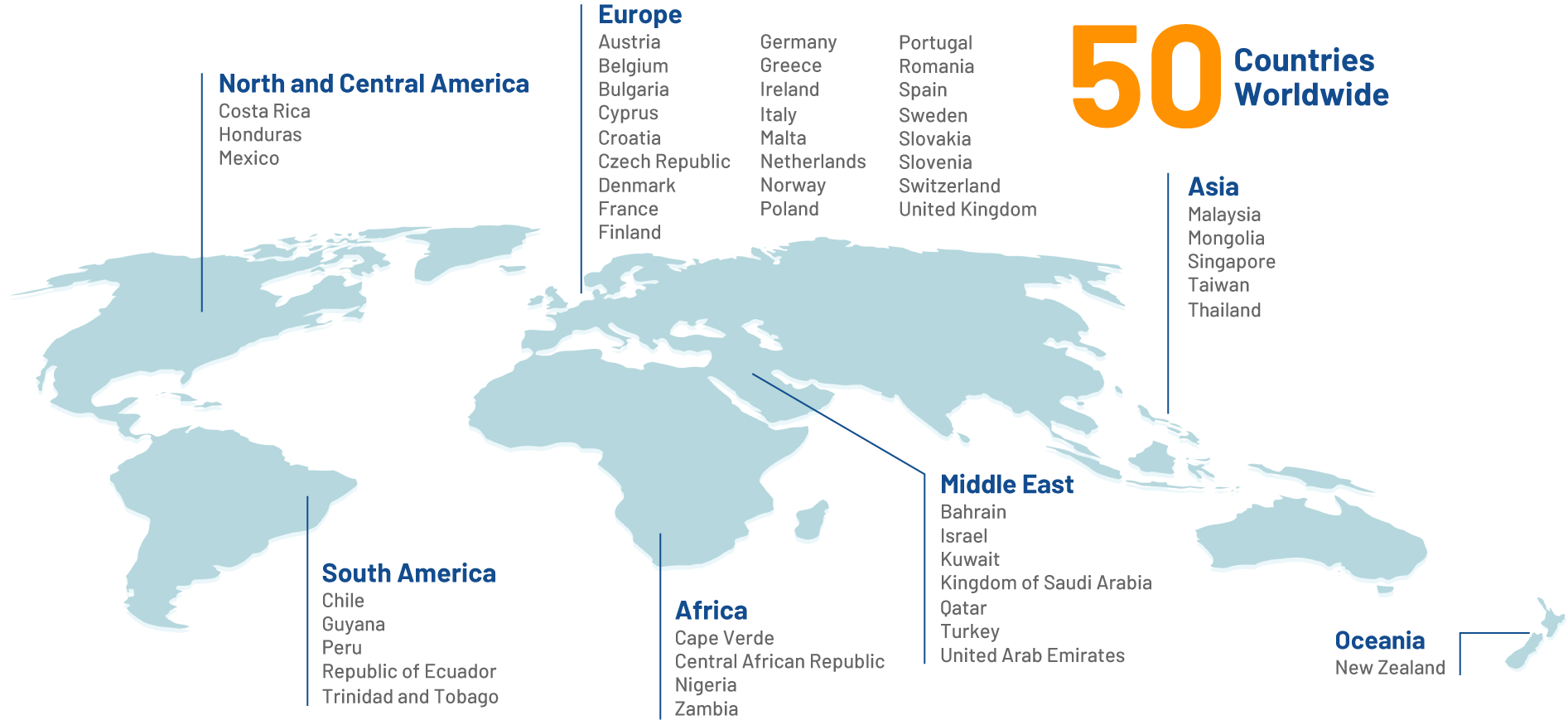
Our Reach

Making a positive impact on the lives of billions of people around the globe
Career


